Which of the Following Has the Lowest Ph
01 М НАО рК - 243 01 M HB pK 455 3D 01 M HCO pK 823 3D 3. онанснунанснан CH2CH3 ortho-ethylheptylcyclopentane meto.

Acids And Bases Ck 12 Foundation
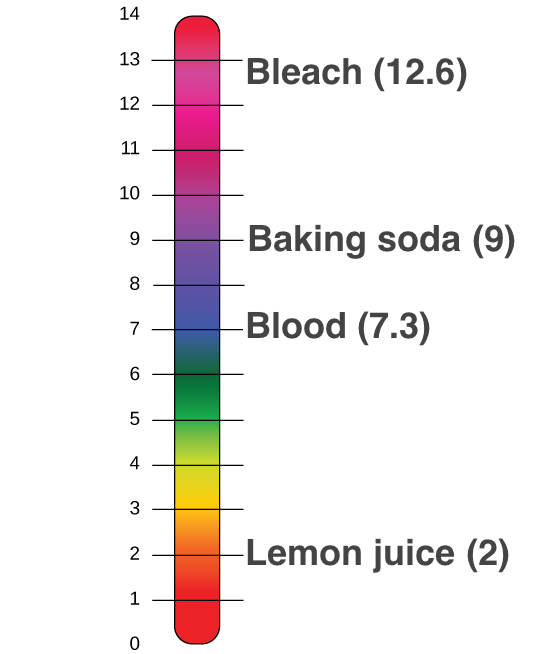
One of the buffers that contribute to pH stability in human blood is carbonic acid H2CO3.

. Carbonic acid is a weak acid that dissociates into a bicarbonate ion HCO3- and a hydrogen ion H. Which of the following solutions would have the lowest pH. 01 M HBO pKa 243.
01 M HBO pka 243 01 MHA pka 455 01 M HMO pKa 823 01 M HST Pka 1189 pure water O HA HST HMO HBO pure water Question 25 Select the correct name for the following compound. It should be noted that the lesser the pH value the stronger is the acid and the higher the pH-value the stronger is the base. So HCl will have lower P.
Blood is slightly alkaline. Watch Video in App Continue on Whatsapp. Since ionisation of HCl ionisation of H 2.
Which of the following solutions has the lowest pH value. Which of the following 10 M solutions would have the lowest pH. This browser does not support the video element.
Every one who is familiar with the stronger acids knows that HNO3 is one of the strongest acid stronger indeed than HNO2. B A solution with a pOH of 210 is very acidic. A 010 M solution of a weak acid HX is.
The weak base within this list is NH3. Assume that they are all 010 M in acid at 25C. Chemistry questions and answers.
ThusH2CO3 HCO3- HIf the pH of the blood drops one would expect. Hence the correct answer is option A. Which of the following body fluids has the lowest pH.
Stomach has lowest pH. Which of the following substances has the lowest pH a plain coffee b household ammonia c plain water d gastric juice. Unshaded spheres represent H atoms and shaded spheres represent A- ions.
The Acidity of the given acids will be in the order 02M HNO3 01M H2SO4 01M HCl 02M CH3-COOH. Which of the following solutions has the lowest pH at 25 o C. Get Answer to any question just click a photo and upload the photo and get the answer completely free UPLOAD PHOTO AND GET THE ANSWER NOW.
Join the 2 Crores Student community now. Asked Sep 3 2019 in Anatomy Physiology by Gordon. Lowest P H corresponds to the strong acid which has very high value of ionization constant.
The stronger the acid the lower the pH since it dissociates more in solution than weaker acids thus making it more acidic and lowering the pH. We know that pH -log H pH. 01 M HMO pKa 823.
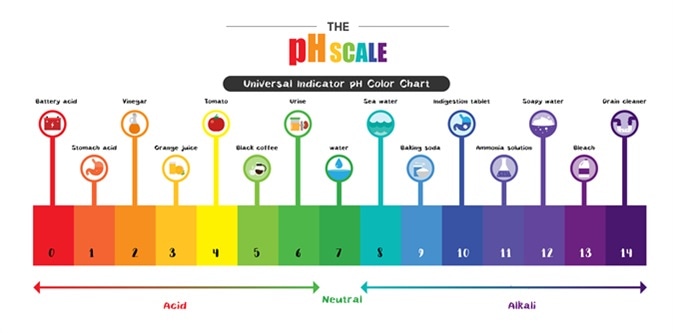
The acid is followed by its Ka value. Which of the following has the lowest pH. Hence the correct option is b- vinegar.
How many times acidic is the substance with lowest pH than the one higher pH. Previous question Next question. D The contents of the stomach.
From all the above options vinegar has the lowest pH-value that means it is the most acidic from the above and the washing soda is most basic. Urine and saliva are slightly acidic. What substance has the lowest pH 0.
Urine while usually acidic rarely falls below pH of 5. Gastric juice is strongly acidic. The stomach contents contain hydrochloric acid secreted by the parietal cells of the gastric glands so typically has a pH of less than 20.
Find solutions to your doubts by just clicking a. Which acid has the lowest pH. Which of the following has minimum pH bile gastric juice saliva pancreatic juice.
17 Which of the following solutions has the lowest pH at 25 o C. Download the Infinity Doubts app now. Saliva is secreted by salivary gland and its pH is about 68.
We know that the stomach contains hydrochloric acid which is capable of dissolving metals. 01 M HA pKa 455. Which of the following could be XBr.
OHCHO2 18 10-4 OHCIO2 11 10-2 HF 35 x 10-4 HCN 49 x 10-10. From all the above options vinegar has the lowest pH-value that means it is the most acidic from the above and the washing soda is most basic. The strong bases within this list are.
You may get confused by the pH range. Answer to Which of the following acids has the lowest pH. Pure water О HD O HAO O pure SolutionInn.
No calculations required A 02 M sodium hydroxide B 02 M hypochlorous acid C 02 M. So ultimately the pH of 02N or 02M HNO3 will be the lowest with a value of 06990. A only solution 1 B only solution 2 C only solution 3 D solutions 1 and 3 Answer.
Which of the following salt have lowest pH. The easiest way to remember pepsins optimum pH is that its present in the stomach. Acids have low pH less than 7.
The following pictures represent solutions that contain a weak acid HA pKa 50 and its potassium salt KA. No calculations required a 02 M sodium hydroxide b 02 M hypochlorous acid c 02 M ammonia d 02 M benzoic acid e pure water 16. Now lets figure out where the acids and bases fall on the pH scale.
So the strongest acid of the list is HNO3 and it has the lowest pH. This browser does not support the video element. Question 24 Which of the following acids has the lowest pH.
01 M HST pKa. Which of the following acids has the lowest pH. To keep watching this video solution for FREE Download our App.
So you should only need to search for the Ka or pKa of HNO2 and HNO3.

No comments for "Which of the Following Has the Lowest Ph"
Post a Comment